
What is Graphene?
Let’s start with a very short story, two physicists were having fun experimenting and they used scotch tape to remove layers of carbon from a lump of Graphite (material used in pencils). Voila, they made Graphene. And this thing got them the Nobel prize in Physics in 2010. Those two physicists were Andre Geim and Konstantin Novoselov. Now it sounds just too simple but there is still a lot to be known about Graphene.

Image credits: Wikipedia
Graphene in simple words is a very thin layer of carbon atoms, and by very thin I mean only one atom thick!! It’s a sheet of carbon atoms in hexagonal lattice. Fun fact: When you write with a pencil there’s a chance you might have accidentally created Graphene!
Let’s get straight to the properties of Graphene to help us understand why it is of such significance.
Properties of Graphene:
- Structure: Graphene is a crystalline allotrope of carbon and is tightly packed in regular hexagonal pattern in one plane.Graphene’s stability is due to its tightly packed carbon atoms and a sp2 orbital hybridisation – a combination of orbitals s, px and py that constitute the σ-bond. The final pz electron makes up the π-bond. These π-bonds are responsible for most of graphene’s notable electronic properties, via the half-filled band that permits free-moving electrons. In simple words sp2 orbital gives graphene its strength and pz electron helps electrons to move easily. It’s actually a perfect 2D structure as its just one atom thick, well in atomic scale its 3D but its the best 2D structure we can get. Something so thin will be obviously very light weight. Apparently less than a gram of graphene sheet can cover an entire football field. And to top it off the structure of Graphene is so strong that it would take an elephant balancing on a sharp pencil to pierce a Graphene sheet with the thickness of Saran Wrap. (This fact about the strength of Graphene sheet is totally mind blowing and hard to digest). In technical terms the tensile strength of Graphene is 130 GPa , for comparison the tensile strength of stainless steel is 860 MPa !!
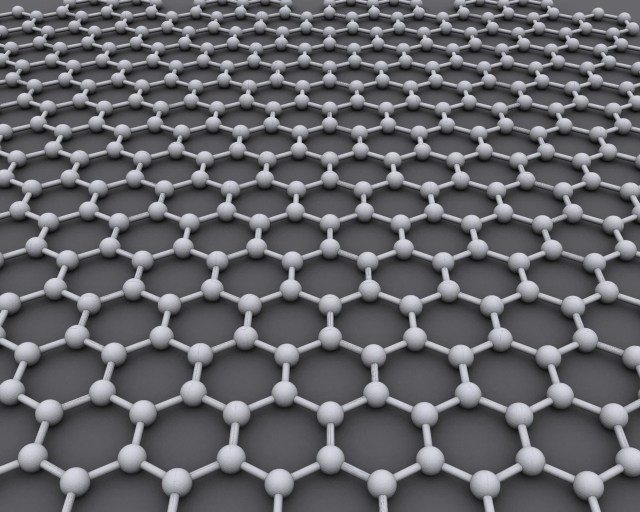
- Thermal Conductivity: Now we know Graphene is super strong very light weight one atom thick sheet of carbon atoms. As if it wasn’t enough Graphene is better at conducting heat than any other material. It is 10 times better at carrying heat than copper.
- Electronic Properties: Graphene is also a very very good conductor of electricity. It has very less resistance due to the uniform flat structure of graphene, so the electrons flow very easily. At room temperature it can conduct electricity faster than any other known material.
- Optical Properties: As Graphene is extremely thin it is almost transparent as one might expect. Infact Graphene transmits about 97% of light which is more than a glass pane.
Summary: So in short Graphene is ultra light, ultra thin, super strong, transparent and a very good conductor of heat and electricity!!

Image credits: www.dailymail.co.uk
Now lets get to the possible applications of this futuristic material.
Applications:
- Graphene is transparent and flexible conductor which is very promising for applications in LEDs, Solar cells, flexible Touchscreens for wearable gadgets, etc.
- Graphene super-capacitors serve as energy storage alternatives to traditional electrolytic batteries. Some advantages are fast charging, long life span and environment friendly production.
- According to Moore’s Law, Silicon transistors are becoming smaller and smaller and hence approaching its limits. Graphene can be an exciting replacement in electronic devices due to its amazing properties and many big companies are working on it.
- Graphene can be used to coat materials to increase their structural strength.
- Such super materials have a direct application is sports. Like Graphene is used in tennis rackets and is said to have better performance than normal rackets. One other application is in Formula 1 cars (Which is already on a whole another level in terms of engineering and technology) or in sports cars. BAC’s 2016 Mono model is said to be made out of graphene as a first of both a street-legal track car and a production car.

Image credits: www.topspeed.com
- The multifunctional nature of graphene means that it is going to have limitless applications we haven’t even thought of yet. It can be used in aerospace applications, motor vehicles, flexible wearable electronic devices, and many applications in medical and biomedical devices.
Inspite of all these benefits of a material of amazing properties whats stopping us from actually using it in the above applications? Well the method used by Andre Geim and Konstantin Novoselov produces very less amount of Graphene and it may not be a perfect single layer of Graphene. The various methods of Graphene production are Mechanical Exfoliation (which means using scotch tape and other adhesives to peel of layers from Graphite), Chemical Vapour Deposition (in which we can produce comparatively big sheets of Graphene than exfoliation), Dispersing graphite in a liquid medium can produce graphene by sonication followed by centrifugation, etc. But all these methods produce less amounts of Graphene and new methods are being developed to reduce defects and production costs. As Graphene sheets are very difficult to make it is one of the most expensive materials on the planet as of now.
Production and development of Graphene is a very active field of research in the field of material science and it is said to be the material of the future. There might be other materials not yet discovered maybe similar or better than Graphene we don’t know!